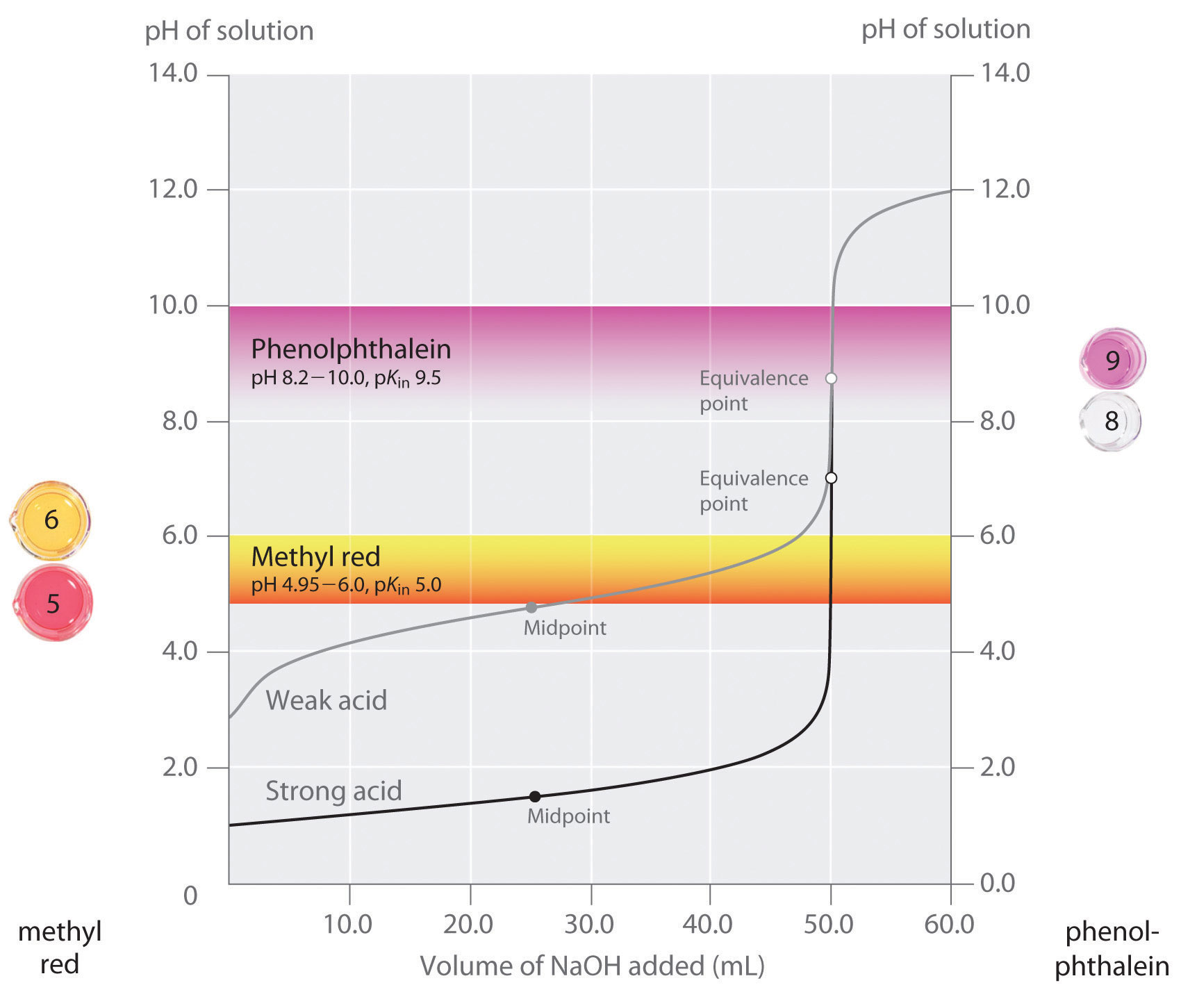
What Is An Indicator In Chemistry Titration . indicators are substances whose solutions change color due to changes in ph. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The indicator changes color when it reaches a critical range of values. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. how titrations work and what you need. Every titration uses the same players: determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution.
from chem.libretexts.org
determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. indicators are substances whose solutions change color due to changes in ph. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. The indicator changes color when it reaches a critical range of values. Every titration uses the same players: how titrations work and what you need. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point.
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts
What Is An Indicator In Chemistry Titration determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. The indicator changes color when it reaches a critical range of values. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Every titration uses the same players: chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. how titrations work and what you need. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. indicators are substances whose solutions change color due to changes in ph.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog What Is An Indicator In Chemistry Titration The indicator changes color when it reaches a critical range of values. Every titration uses the same players: They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by. What Is An Indicator In Chemistry Titration.
From mungfali.com
Acid Base Titration Indicator What Is An Indicator In Chemistry Titration revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. The indicator changes color when it reaches a critical range of values. how titrations work and what you need. Every titration uses the same players: we have stated that a good indicator should. What Is An Indicator In Chemistry Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators What Is An Indicator In Chemistry Titration how titrations work and what you need. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Every titration uses the same players: we have stated. What Is An Indicator In Chemistry Titration.
From printablevozljevrc.z21.web.core.windows.net
How To Do Titrations In Chemistry What Is An Indicator In Chemistry Titration chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. how titrations work and what you need. They are usually weak acids or bases, but their conjugate base or acid forms. What Is An Indicator In Chemistry Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog What Is An Indicator In Chemistry Titration determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence. What Is An Indicator In Chemistry Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co What Is An Indicator In Chemistry Titration indicators are substances whose solutions change color due to changes in ph. The indicator changes color when it reaches a critical range of values. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali. What Is An Indicator In Chemistry Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog What Is An Indicator In Chemistry Titration revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. how titrations work and what you need. The indicator changes color when it reaches a critical range of values. we have stated that a good indicator should have a pkin value that is. What Is An Indicator In Chemistry Titration.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry What Is An Indicator In Chemistry Titration determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Every titration uses the same players: revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. how titrations work and what you need. chemical indicator,. What Is An Indicator In Chemistry Titration.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog What Is An Indicator In Chemistry Titration revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The indicator changes color when it reaches a critical range of. What Is An Indicator In Chemistry Titration.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog What Is An Indicator In Chemistry Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. indicators are substances whose solutions change color due to changes in ph. Every titration uses the same players: revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written. What Is An Indicator In Chemistry Titration.
From dxoyufult.blob.core.windows.net
Titration Analysis Cation at Sean Eubanks blog What Is An Indicator In Chemistry Titration chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at. What Is An Indicator In Chemistry Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic What Is An Indicator In Chemistry Titration how titrations work and what you need. indicators are substances whose solutions change color due to changes in ph. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. They. What Is An Indicator In Chemistry Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators What Is An Indicator In Chemistry Titration determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. The indicator changes color when it reaches a critical range of values. Every titration uses the same players: chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. What Is An Indicator In Chemistry Titration.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS What Is An Indicator In Chemistry Titration determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. The indicator changes color when it reaches a critical range of values. how titrations work and what you need. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a. What Is An Indicator In Chemistry Titration.
From mmerevise.co.uk
Titrations and Uncertainties MME What Is An Indicator In Chemistry Titration chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. revision notes on 1.7.13 indicators used in titration for the cie a level chemistry syllabus, written by the chemistry experts at. What Is An Indicator In Chemistry Titration.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE What Is An Indicator In Chemistry Titration we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. The indicator changes color when it reaches a critical range of values. Every titration uses the same players: determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration.. What Is An Indicator In Chemistry Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit What Is An Indicator In Chemistry Titration chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration of a chemical species, such as an acid or an alkali in a solution. The indicator changes color when it reaches a critical range of values. Every titration uses the same players: we have stated. What Is An Indicator In Chemistry Titration.
From ar.inspiredpencil.com
Titration Setup Diagram What Is An Indicator In Chemistry Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. indicators are substances whose solutions change color due to changes in ph. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. What Is An Indicator In Chemistry Titration.