What Is Liquid Gas Mixture . Lpg stands for liquefied petroleum gas and the term is used to describe two natural gas liquids: One of the properties of gases is that they mix with each other. This page deals with raoult's law and how it applies to mixtures of two volatile liquids. Liquefied petroleum gas, any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. For a mixture of two ideal gases, \(a\). When they do so, they become a solution—a homogeneous mixture. Within the pipeline, these products can be either a gas, or two phase gas/ liquid mixture or, if above their critical temperature or. It covers cases where the two liquids are entirely miscible in all proportions to. Propane and butane, or a mix of the two. Some of the properties of gas mixtures are easy to. The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases.
from www.shutterstock.com
Lpg stands for liquefied petroleum gas and the term is used to describe two natural gas liquids: When they do so, they become a solution—a homogeneous mixture. Some of the properties of gas mixtures are easy to. Liquefied petroleum gas, any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. One of the properties of gases is that they mix with each other. It covers cases where the two liquids are entirely miscible in all proportions to. This page deals with raoult's law and how it applies to mixtures of two volatile liquids. Propane and butane, or a mix of the two. The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. Within the pipeline, these products can be either a gas, or two phase gas/ liquid mixture or, if above their critical temperature or.
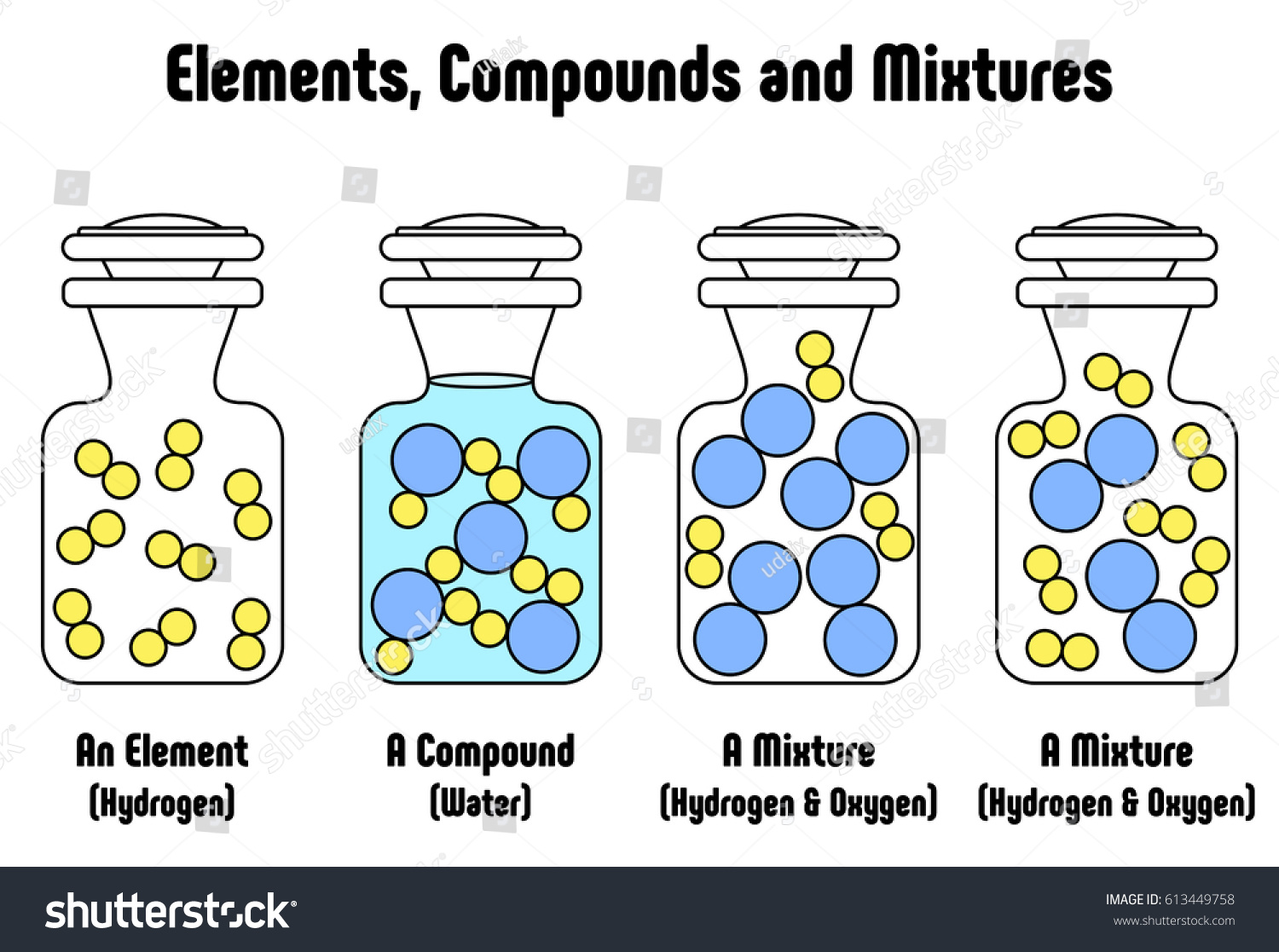
Different Between Elements Compounds Mixtures Example Stock
What Is Liquid Gas Mixture For a mixture of two ideal gases, \(a\). This page deals with raoult's law and how it applies to mixtures of two volatile liquids. Liquefied petroleum gas, any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. Lpg stands for liquefied petroleum gas and the term is used to describe two natural gas liquids: Some of the properties of gas mixtures are easy to. When they do so, they become a solution—a homogeneous mixture. The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. For a mixture of two ideal gases, \(a\). It covers cases where the two liquids are entirely miscible in all proportions to. Propane and butane, or a mix of the two. Within the pipeline, these products can be either a gas, or two phase gas/ liquid mixture or, if above their critical temperature or. One of the properties of gases is that they mix with each other.
From www.dreamstime.com
States of Matter. Vector Circles Infographic Illustration Stock Vector What Is Liquid Gas Mixture Within the pipeline, these products can be either a gas, or two phase gas/ liquid mixture or, if above their critical temperature or. One of the properties of gases is that they mix with each other. This page deals with raoult's law and how it applies to mixtures of two volatile liquids. Lpg stands for liquefied petroleum gas and the. What Is Liquid Gas Mixture.
From lokasinmemo.weebly.com
Solid liquid gas lokasinmemo What Is Liquid Gas Mixture Propane and butane, or a mix of the two. One of the properties of gases is that they mix with each other. Liquefied petroleum gas, any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. Within the pipeline, these products can be either a gas, or two phase gas/ liquid mixture or, if above their critical. What Is Liquid Gas Mixture.
From space.ravulacharan.com
What is the difference between liquidfueled and solidfueled rockets? What Is Liquid Gas Mixture Propane and butane, or a mix of the two. One of the properties of gases is that they mix with each other. Liquefied petroleum gas, any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. Some of the properties of gas mixtures are easy to. The total pressure of a mixture of gases is the sum. What Is Liquid Gas Mixture.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 27, pt 1 of 3 Properties What Is Liquid Gas Mixture One of the properties of gases is that they mix with each other. Within the pipeline, these products can be either a gas, or two phase gas/ liquid mixture or, if above their critical temperature or. For a mixture of two ideal gases, \(a\). The total pressure of a mixture of gases is the sum of the partial pressures of. What Is Liquid Gas Mixture.
From www.youtube.com
Science Lesson 4 Mixing Solids into Liquids YouTube What Is Liquid Gas Mixture It covers cases where the two liquids are entirely miscible in all proportions to. One of the properties of gases is that they mix with each other. Lpg stands for liquefied petroleum gas and the term is used to describe two natural gas liquids: When they do so, they become a solution—a homogeneous mixture. Liquefied petroleum gas, any of several. What Is Liquid Gas Mixture.
From www.coursehero.com
[Solved] A gaseous mixture of O2 and N2 contains 38.8 nitrogen by What Is Liquid Gas Mixture Propane and butane, or a mix of the two. Lpg stands for liquefied petroleum gas and the term is used to describe two natural gas liquids: When they do so, they become a solution—a homogeneous mixture. The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. For a mixture of two. What Is Liquid Gas Mixture.
From www.theengineersperspectives.com
Gas Absorption Packed Column The Engineer's Perspective What Is Liquid Gas Mixture This page deals with raoult's law and how it applies to mixtures of two volatile liquids. The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. Lpg stands for liquefied petroleum gas and the term is used to describe two natural gas liquids: Propane and butane, or a mix of the. What Is Liquid Gas Mixture.
From mavink.com
Gas Bottle Sizes Chart What Is Liquid Gas Mixture Within the pipeline, these products can be either a gas, or two phase gas/ liquid mixture or, if above their critical temperature or. Liquefied petroleum gas, any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. For a mixture of two ideal gases, \(a\). Some of the properties of gas mixtures are easy to. It covers. What Is Liquid Gas Mixture.
From www.youtube.com
Liquid Gas (Mixture) Separation & Chromatography l Chemistry l Grade 9 What Is Liquid Gas Mixture One of the properties of gases is that they mix with each other. When they do so, they become a solution—a homogeneous mixture. Liquefied petroleum gas, any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. Propane and butane, or a mix of the two. Lpg stands for liquefied petroleum gas and the term is used. What Is Liquid Gas Mixture.
From www.youtube.com
[7] finding density of a mixture YouTube What Is Liquid Gas Mixture The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. For a mixture of two ideal gases, \(a\). One of the properties of gases is that they mix with each other. Lpg stands for liquefied petroleum gas and the term is used to describe two natural gas liquids: Some of the. What Is Liquid Gas Mixture.
From www.shutterstock.com
Different Between Elements Compounds Mixtures Example Stock What Is Liquid Gas Mixture Lpg stands for liquefied petroleum gas and the term is used to describe two natural gas liquids: It covers cases where the two liquids are entirely miscible in all proportions to. One of the properties of gases is that they mix with each other. For a mixture of two ideal gases, \(a\). Some of the properties of gas mixtures are. What Is Liquid Gas Mixture.
From www.gasrebate.net
Properties Of Solids Liquids Gases Compared Teachoo Science What Is Liquid Gas Mixture Within the pipeline, these products can be either a gas, or two phase gas/ liquid mixture or, if above their critical temperature or. Propane and butane, or a mix of the two. It covers cases where the two liquids are entirely miscible in all proportions to. The total pressure of a mixture of gases is the sum of the partial. What Is Liquid Gas Mixture.
From www.shanleypump.com
Standard and Multiphase GasLiquid Mixture Centrifugal Pumps What Is Liquid Gas Mixture When they do so, they become a solution—a homogeneous mixture. For a mixture of two ideal gases, \(a\). Some of the properties of gas mixtures are easy to. It covers cases where the two liquids are entirely miscible in all proportions to. Liquefied petroleum gas, any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. Within. What Is Liquid Gas Mixture.
From www.yourdictionary.com
Examples of Heterogeneous Mixtures Types Made Simple YourDictionary What Is Liquid Gas Mixture One of the properties of gases is that they mix with each other. When they do so, they become a solution—a homogeneous mixture. The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. Liquefied petroleum gas, any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. It. What Is Liquid Gas Mixture.
From courses.lumenlearning.com
Stoichiometry of Gaseous Substances, Mixtures, and Reactions What Is Liquid Gas Mixture One of the properties of gases is that they mix with each other. This page deals with raoult's law and how it applies to mixtures of two volatile liquids. The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. It covers cases where the two liquids are entirely miscible in all. What Is Liquid Gas Mixture.
From www.bartleby.com
Answered The molecular view of a gaseous mixture… bartleby What Is Liquid Gas Mixture Liquefied petroleum gas, any of several liquid mixtures of the volatile hydrocarbons propene, propane, butene, and butane. When they do so, they become a solution—a homogeneous mixture. The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. It covers cases where the two liquids are entirely miscible in all proportions to.. What Is Liquid Gas Mixture.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law What Is Liquid Gas Mixture For a mixture of two ideal gases, \(a\). Some of the properties of gas mixtures are easy to. The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. One of the properties of gases is that they mix with each other. Within the pipeline, these products can be either a gas,. What Is Liquid Gas Mixture.
From byjus.com
A mixture of two immiscible liquid A and B is distilled under What Is Liquid Gas Mixture When they do so, they become a solution—a homogeneous mixture. It covers cases where the two liquids are entirely miscible in all proportions to. For a mixture of two ideal gases, \(a\). Propane and butane, or a mix of the two. The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases.. What Is Liquid Gas Mixture.